Functional Groups This is the chemical structure of the ethyl functional group. The term ester was coined by the German chemist Leopold Gmelin in 1848. A common ester - ethyl ethanoate.
The general formula of a hemiacetal is R 1 R 2 COHOR where R 1 or R 2 is often hydrogen and R bonded to O is not hydrogen.
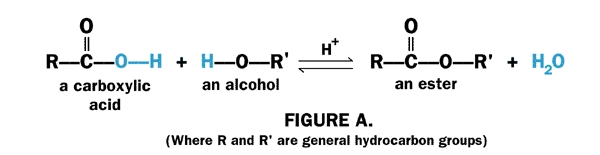
An ester is characterized by the orientation and bonding of the atoms shown where R and R are both carbon-initiated chains of varying length also known as alkyl groups. The general formula of a hemiacetal is R 1 R 2 COHOR where R 1 or R 2 is often hydrogen and R bonded to O is not hydrogen. Ester names are derived from the parent alcohol and the parent acid where the latter may be organic or inorganic. The reaction of salicylic acid C 6 H 4 OHCO 2 H and methanol CH 3 OH forms this ester.